
|

|
Posted 14 November 2023 AM
Recent expansions in the populations of eligible patients have seen a couple of drugs surge dramatically year-on-year.
Analysis by Pharma in Focus has revealed Janssen's prostate cancer treatment, Erlyand, saw explosive year-on-year growth last quarter, earning more than 70 times what it did in the third quarter of 2022 in pre-rebate R/PBS reimbursements.
A year ago, Erlyand was listed for patients with non-metastatic castration resistant prostate cancer, for which it gained a PBAC recommendation in November 2021. At that same meeting it sought, and failed to get, a recommendation for the treatment of metastatic hormone sensitive prostate cancer (mHSPC) in patients who have low volume disease, or high volume disease and who are unsuitable for chemotherapy.
Janssen tried again in the July 2022 PBAC meeting, and this time garnered a recommendation for mHSPC in patients irrespective of disease volume or suitability for treatment with docetaxel, a larger population than it had sought.
 |
PBAC noted a price reduction was required for Erlyand to be cost effective in this indication, which could explain why it didn't list until June this year. It was worth the wait for Janssen though, with the new indication earning $3.84 million, just over two-thirds of total reimbursements.
Mind you, the first listing also increased from $362,296 in Q2 to $1,852,167 in Q3, a 411 per cent increase.
Antengene's anti-cancer drug Xpovio also benefited from a June PBS expansion. It was first listed in September last year, and quickly began earning above $900,000 a quarter.
With listing for an expanded population in June this year, it grew another 37 per cent compared to Q2.
BeiGene's BTK inhibitor Brukinsa has been growing steadily since listing at the beginning of 2022, and scored another couple of indications in September this year, which alone added $776,000 to its total for the quarter. It's tipped to disrupt the blood cancer market.
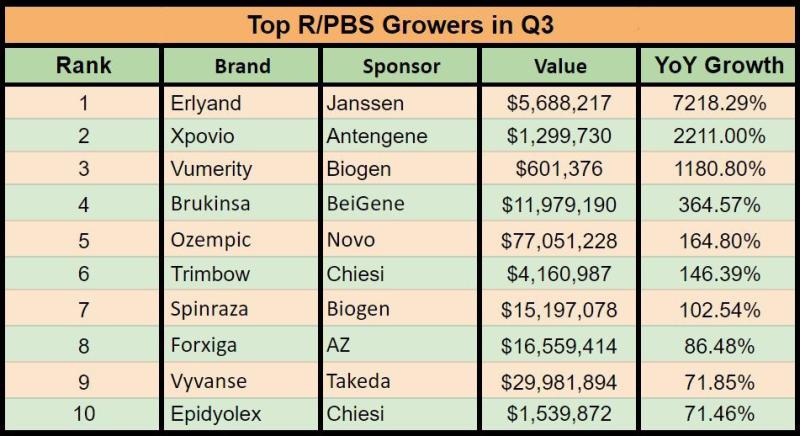 |
Novo Nordisk's diabetes drug Ozempic saw solid growth as the company works to keep up with demand. The drug saw the highest growth in dollar terms, adding $48 million to its reimbursements compared to a year earlier.
Biogen's spinal muscular atrophy treatment Spinraza more than doubled its reimbursements over the year, after scoring two expansions in the second half of 2022. In August 2022 it was expanded to include adults with 5q SMA and symptom onset prior to their 19th birthday, and a month later expanded again to include treatment of all paediatric patients with Type III SMA.
Chiesi's Trimbow scored an additional listing at the start of this year for the maintenance treatment of asthma, although without the additional listing its reimbursements would have almost doubled, keeping it on the list.
Takeda's ADHD treatment Vyvanse was growing steadily as more people were diagnosed with ADHD, and changes to prescription restrictions in May this year have given it another boost. Chiesi's oral cannabidiol Epidyolex won reimbursement for Lennox-Gastaut syndrome in June this year, while Biogen's multiple sclerosis drug Vumerity was first listed in August last year.
James Quintana Pearce
If you were passed this article by a colleague, chances are you've missed other important Pharma in Focus articles and features.
To find out more, go to www.pharmainfocus.com.au and sign up for a FREE Full Text trial
Pharma in Focus
Australia's most trusted source of pharma news
© Copyright Lush Media. End of News - printed 04 December 2024