
|

|
Posted 3 June 2024 AM
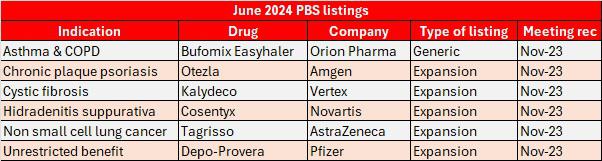
There have been six additions to the PBS this month, ranging from a generic to noteworthy expansions that could disrupt the market.
All the drugs listed were recommended at the PBAC's November 2023 meeting, including Novartis' Cosentyx which has set up a battle with AbbVie's Humira and biosimilars of the drug in becoming the second biologic disease modifying anti-rheumatic drug subsidised in Australia for hidradenitis suppurativa.
Cosentyx made Novartis $99.3 million in pre-rebate PBS benefits in 2023 treating plaque psoriasis, psoriatic arthritis, non-radiographic axial spondylarthritis, and ankylosing spondylitis.
In the oncology field, AstraZeneca's Tagrisso has been expanded for the treatment of Stage IB to IIIA EGFR mutation positive non-small cell lung cancer. The kinase inhibitor earned the company over $153 million in pre-rebate PBS benefits last year.
"Hundreds of Australians with early-stage non-small cell lung cancer may now be eligible for reimbursed access to Tagrisso," AstraZeneca ANZ Country President Ben McDonald said.
"With the national lung cancer screening program to begin next year, patients need more treatment options for earlier stage diagnosis.
 |
"Thank you to the Australian clinicians and patients that have been involved in the clinical trials for this indication, working tirelessly to have this treatment available on the PBS for this type of lung cancer."
Vertex's Kalydeco has also been expanded for patients aged four months or older who have a G551D or other gating (Class III) mutation on the CFTR gene. Additionally, patients aged four months or older with at least one non-gating mutation on the CFTR gene that is responsive to Kalydeco potentiation based on clinical or in vitro data are also eligible.
"We are delighted that the Australian Government has expanded the reimbursement of Kalydeco. It marks further progress towards our mission of providing treatment for all people living with CF regardless of age or genotype," Vertex Senior Country Manager ANZ, Sabrina Barbic said.
Cystic Fibrosis Australia has hailed the decision as a "beacon of hope for the CF community" with around 95 patients now eligible for a CFTR modulator for the first time under this expansion.
However, the strong uptake of Vertex's triple combination CF drug Trikafta has reduced the earnings of the company's three other CF drugs, including Kalydeco which saw earnings fall from $47.9 million in 2022 to $21.8 million in 2023.
Other June listings include Orion Pharma's asthma and COPD inhaler Bufomix Easyhaler targeting AstraZeneca's Symbicort Turbuhaler
There has also been a change in the treatment criteria for Amgen's Otezla in severe chronic plaque psoriasis allowing rheumatologists and general physicians to initiate treatment (in addition to dermatologists) plus rheumatology registrars in consultation with one of the named practitioner types.
Rounding up the list is Pfizer's pre-filled syringe form of its injectable hormonal medication Depo-Provera, which has been listed under the same circumstances as the currently listed injection, vial forms Depo-Provera and Depo-Ralovera.
All six drugs were among Pharma in Focus' predictions for potential June listings with Juniper's Ledaga, Apsen's Slenyto, AstraZeneca's Lynparza, and MSD's Stromectol missing out.
 |
Tiffany Walker
If you were passed this article by a colleague, chances are you've missed other important Pharma in Focus articles and features.
To find out more, go to www.pharmainfocus.com.au and sign up for a FREE Full Text trial
Pharma in Focus
Australia's most trusted source of pharma news
© Copyright Lush Media. End of News - printed 13 January 2025